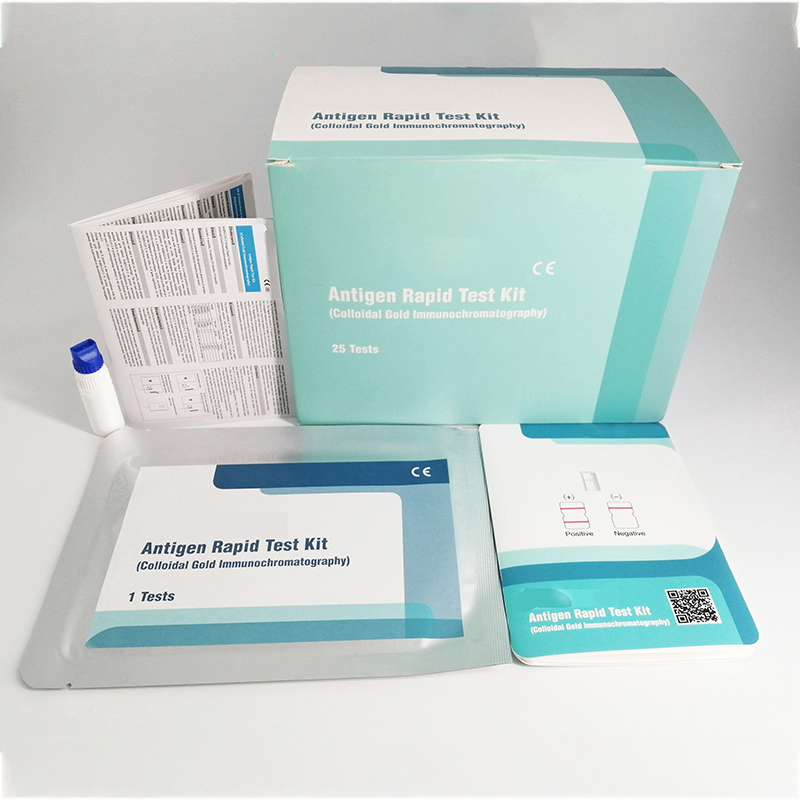
 Infectious Disease/Virus Antigen Test Kit (Colloidal Gold lmmunochromatography) COVID-19 Antigen Rapid Test is a rapid device for the qualitative detection of SARS-CoV-2 antigens in nasopharyngeal and nasal.
Infectious Disease/Virus Antigen Test Kit (Colloidal Gold lmmunochromatography) COVID-19 Antigen Rapid Test is a rapid device for the qualitative detection of SARS-CoV-2 antigens in nasopharyngeal and nasal. 
 Infectious Disease/Virus Antigen Test Kit (Colloidal Gold lmmunochromatography) COVID-19 Antigen Rapid Test is a rapid device for the qualitative detection of SARS-CoV-2 antigens in nasopharyngeal and nasal.
Infectious Disease/Virus Antigen Test Kit (Colloidal Gold lmmunochromatography) COVID-19 Antigen Rapid Test is a rapid device for the qualitative detection of SARS-CoV-2 antigens in nasopharyngeal and nasal.